How does the CardioMEMS technology work?
The CardioMEMS™ HF System uses a miniaturized, wireless monitoring sensor that is implanted in the pulmonary artery during a minimally invasive procedure to directly measure PA pressure. The system allows patients to transmit PA pressure data from their homes to their health care providers allowing for personalized and proactive management to reduce the likelihood of hospitalization.
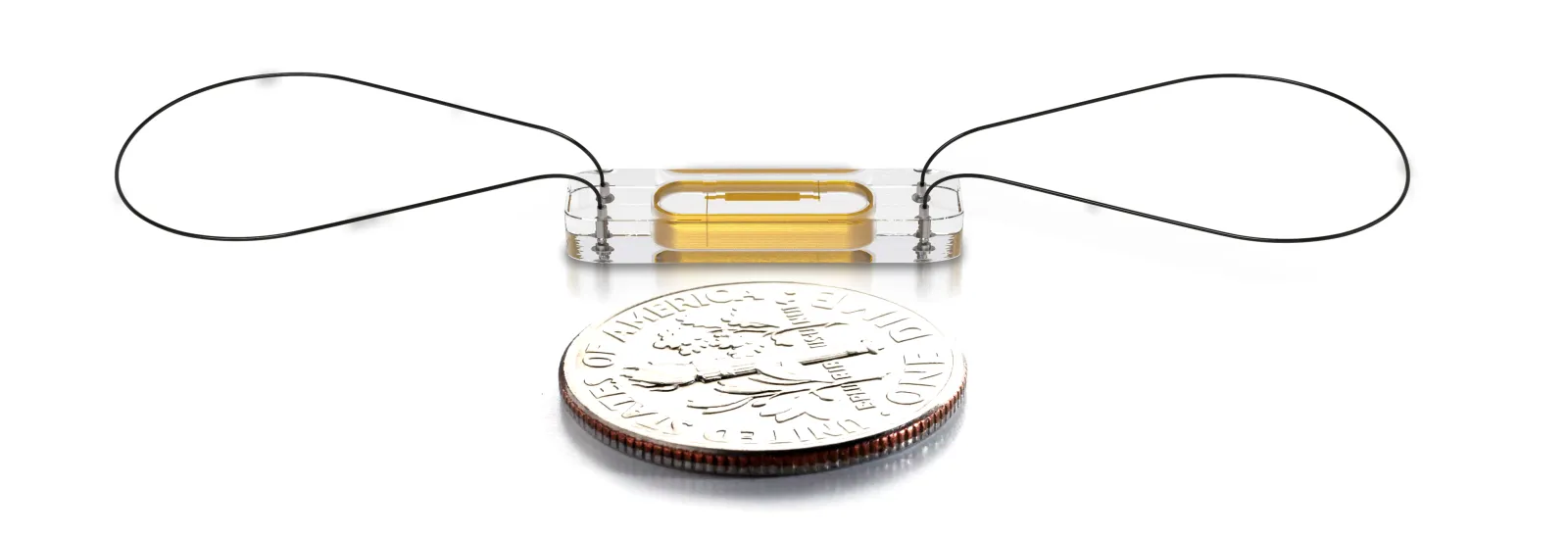
The implantable sensor is a completely sealed capsule that uses microelectromechanical systems (MEMS) technology, which allows the creation of sensors with measurement stability and energy efficiency. All of the sensor components are made of materials that have been chosen for their durability, robustness, biocompatibility, and insensitivity to changes in body chemistry or biology. The sensor is powered by radio frequency (RF) energy. It is implanted into the pulmonary artery using minimally invasive techniques via a catheter and is designed to last the lifetime of the patient.
The sensor does not have a battery or leads and is very small. Once implanted, the sensor wirelessly sends pressure readings to the external patient electronic system. There is no pain or sensation for the patient during the readings. The electronics transmit the readings to a secure website where it can be seen by the patient's clinician.
The external measurement system wirelessly tracks frequency and uses it to determine the pressure in the pulmonary artery.
At home, HF patients use a portable electronic unit and a special pillow containing an antenna to take daily sensor readings. This is a simple process that takes only a few minutes. The patient's electronic unit is turned on and the patient lies on the pillow. The electronic unit uses audible signals telling the patient to press the button to initiate a reading. The pressure readings are then wirelessly transmitted to a secure website.
Clinicians access patients' pressure readings and trending data transmissions using the patient management website, providing vaulable clinical insight for guiding treatment decisions. Automated alerts will be sent to health care providers if pressure readings fall outside of prespecified ranges.
What is the potential impact of the CardioMEMS HF system?
The CardioMEMS HF System is the first and only FDA-approved HF monitoring device that has been proven to significantly reduce hospital admissions and improve quality of life in NYHA class III HF patients who have been hospitalized in the previous 12 months. Nearly 6 million Americans have HF with 900,000 new cases diagnosed each year.
What is the labeled indication for the CardioMEMS HF system?
The CardioMEMS HF System is indicated for wirelessly measuring and monitoring PA pressure and heart rate in New York Heart Association (NYHA) Class III heart failure patients who have been hospitalized for heart failure in the previous year. The hemodynamic data are used by physicians for heart failure management and with the goal of reducing heart failure hospitalizations.
The CardioMEMS HF System is used by the physician in the hospital or office setting to obtain and review PA pressure measurements. The CardioMEMS HF System is used by the patient in the home or other remote location to wirelessly obtain and send hemodynamic and PA pressure measurements to a secure database for review and evaluation by the patient's physician.

